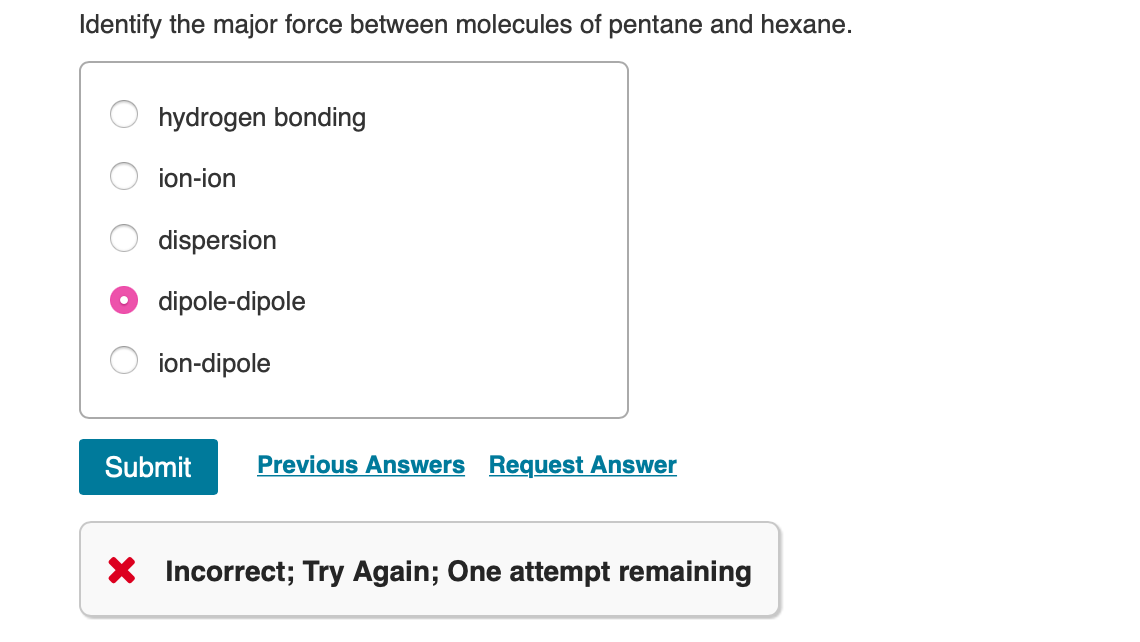
Identify the Major Force Between Molecules of Pentane and Hexane.
Hydrogen bonding d ion-iorn e ion-dipole Which of the following compounds will be most soluble in ethanol СНСНОну a. Major forces between pentane and hexane are discussed below.

Solved 1 Define Solubility A A Solid That Does Not Chegg Com
Asked Aug 18 2019 in Chemistry by Alyssa.
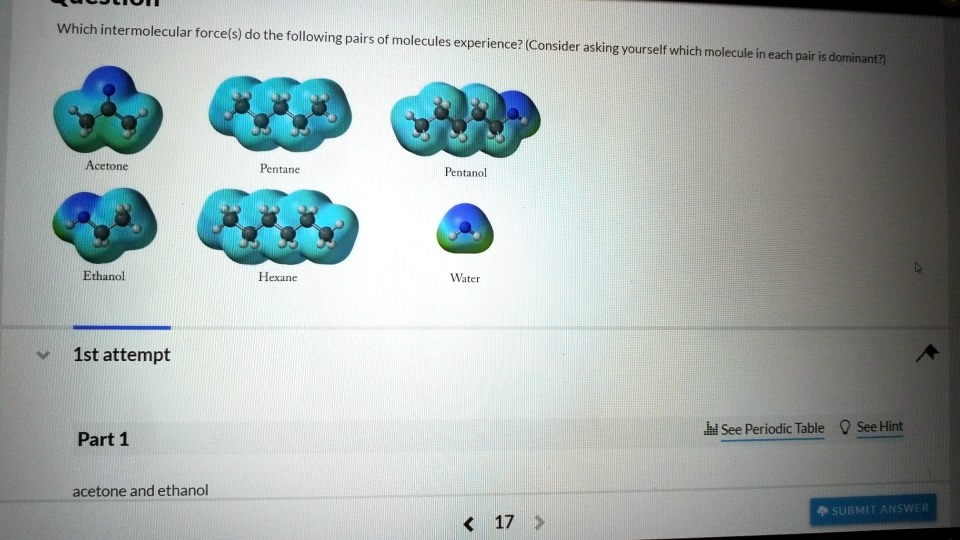
. Give the major force between ethanol and rubbing alcohol. A dipole-dipole B dispersion C hydrogen bonding D ion-ion E ion-dipole 5 If two substances are soluble in all proportions then they are A miscible B nonpolar C immiscible D neutral E polar. None of these compounds should be soluble in ethanol.
Give the major force between acetone and chloroform. Predict the electron geometry. Region with excess electron density has partial - charge.
Hexane CHCH CHCH-CH CHs e. Relative melting point MP boiling point BP and vapor pressure VP can be predicted by comparing molar mass MM. Hexane heptane and pentane are nonpolar.
Therefore the only intermolecular force. Solution for Identify the compound with covalent bonds. Answer the following statement true T or false F.
Pentane and hexane both have london dispersion forces. Hydrogen bonding ion-ion dispersion dipole-dipole ion-dipole Submit Previous Answers Request Answer X Incorrect. The boiling point of the compounds depend on the number of carbons in them.
So lets get. Identify the major force between molecules of pentane and hexane. Select True or False.
Predict the molecule geometry. Dispersion dipole-dipole hydrogen bonding ion-ion ion-dipole. Now go to start search for Run Adeona Recovery.
A molecule has 2 double bonds on the central atom and no lone pairs. Inter molecular forces are the attractions between molecules which determine many of the physical properties of a substance. Molecules with hydrogen bonding are more volatile than compounds with dipole-dipole forces.
Identify the major force between molecules of pentane. Firstly the only bond is C-H which is non-polar due to carbon and hydrogen having very similar electronegativities secondly hexane is symetric so any polarity in the molecule would cancel out. What is the mole fraction of I2 in a solution made by dissolving 556 g of I2 in 245 g of hexane C6H14.
These forces are the result of the movement of electrons which cause slight polar moments. The strengths of these attractive forces vary widely though usually the IMFs between small molecules are weak compared to the intramolecular forces that bond. 1 illustrates these different molecular forces.
- All molecules and atoms will have them. BA Chemistry University of Cambridge 2020 Updated 4 years ago. An intermolecular attraction force that exists between all molecules.
Identify the major force between molecules of pentane and hexane. Flower colours of red pink blue and. Hexane is a non-polar molecule.
Identify the major force between molecules of pentane. This software can also take the picture of the culprit or the thief. Identify the major force between molecules of pentane and hexane.
A solution made of pentane and hexane has a mole fraction X 0250 of pentane. Rank these compounds by boiling point- Pentane Propane and Hexane. When it opensopen the file.
Because of this the only intermolecular force present with these nonpolar covalent molecules are London dispersion. Hexane has 6 Pentane has 5 and Propane has 3 carbons. The properties of liquids are intermediate between those of gases and solids but are more similar to solids.
View the full answer. Identify the major force between molecules of pentane and hexane. The major force between the two compounds containing -OH -NH and F-H bonds is hydrogen bonding.
Identify the major force between molecules of pentane. As Hexane is with 6 carbons it has highest boiling point and next Pentane with 5 and lowest Propane with 3. Both pentane and hexane are symmetrical structures meaning the dipole moment is zero.
Ethylene glycol HOCH CH OH d. Pentane and hexane both have London-dispersion forces as their dominant intermolecular force. - Generally very weak when their molecular mass increases so does their strength.
Asked Aug 7 2019 in Chemistry by Mizzthang. A LiBr B Mg C NaI D CH4 E Ar. Identify the compounds that are soluble in both water and hexane.
Therefore the dominant intermolecular forces in hexane heptane and pentane are induced-dipole induced dipole forces. A dipole-dipole B dispersion C hydrogen bonding D ion-ion E ion-dipole 4 Identify the major force between molecules of pentane and hexane. In contrast to intramolecular forces such as the covalent bonds that hold atoms together in molecules and polyatomic ions intermolecular forces hold molecules together in a liquid or solidIntermolecular forces are generally much weaker than covalent.
Give the major force in NaCl solution. Hexane is the solute. Correspondingly does pentane have hydrogen bonding.
00715 0133 0154 00770. Identify the intermolecular force or forces that predominate in Al2O3 check all that apply Group of answer choices 1. Identify the major force between molecules of pentane.
Group of answer choices. This is down to 2 factors. Acetone CH COCHs c.
Ethylene glycol HOCH2CH2OH c.
Solved Identify The Major Force Between Molecules Of Pentane Chegg Com

Solved Ultiple Choice Ntify The Choice That Best Completes Chegg Com

Solved Which Intermolecular Force S Do The Following Pairs Of Molecules Experience Consider Asking Yoursel Which Molecule In Each Pair Dominantz Acetone Pentane Fentanol Ethanol Hexane Water Ist Attempt See Periodic Table See

No comments for "Identify the Major Force Between Molecules of Pentane and Hexane."
Post a Comment